-
How to Watch Aston Villa vs. Newcastle: Live Stream Premier League, TV Channel - 39 mins ago
-
2025 NBA playoffs: 7 postseason betting thoughts - 42 mins ago
-
Putin announces an Easter truce amid conflict in Ukraine - 51 mins ago
-
Jeff Bridges Wants to Return to Most Beloved Role - about 1 hour ago
-
Royals vs. Tigers Highlights | MLB on FOX - about 1 hour ago
-
Israeli strikes on Gaza kill more than 90 people in 48 hours, Palestinians say - 2 hours ago
-
Tomato Price Warning Issued Over Trump Tariffs - 2 hours ago
-
Mariners vs. Blue JaysHighlights | MLB on FOX - 2 hours ago
-
How to Watch Everton FC vs. Manchester City: Live Stream Premier League, TV Channel - 3 hours ago
-
‘Real Housewives’ star Teddi Mellencamp breaks down over lost time with her kids - 3 hours ago
Parkinson’s Disease Reversed by Brain-Stimulating Nanoparticles
Injections of nanoparticles into the brain could reverse the symptoms of Parkinson’s disease in a less invasive manner than existing treatments, reducing side effects like anxiety, cognitive decline and depression.
This is the conclusion of researchers from China who have shown that injections of the particle into the brains of mice — and their activation with lasers — can reduce the characteristic movement-related symptoms of the disease.
The second-most-common neurodegenerative disorder, Parkinson’s disease manifests via such motor problems as impaired balance, slow movement, muscle stiffness and tremors. According to the Parkinson’s Foundation, nearly a million Americans are living with Parkinson’s disease—a figure expected to rise to 1.2 million by the decade’s end.
Chinnapong/iStock / Getty Images Plus
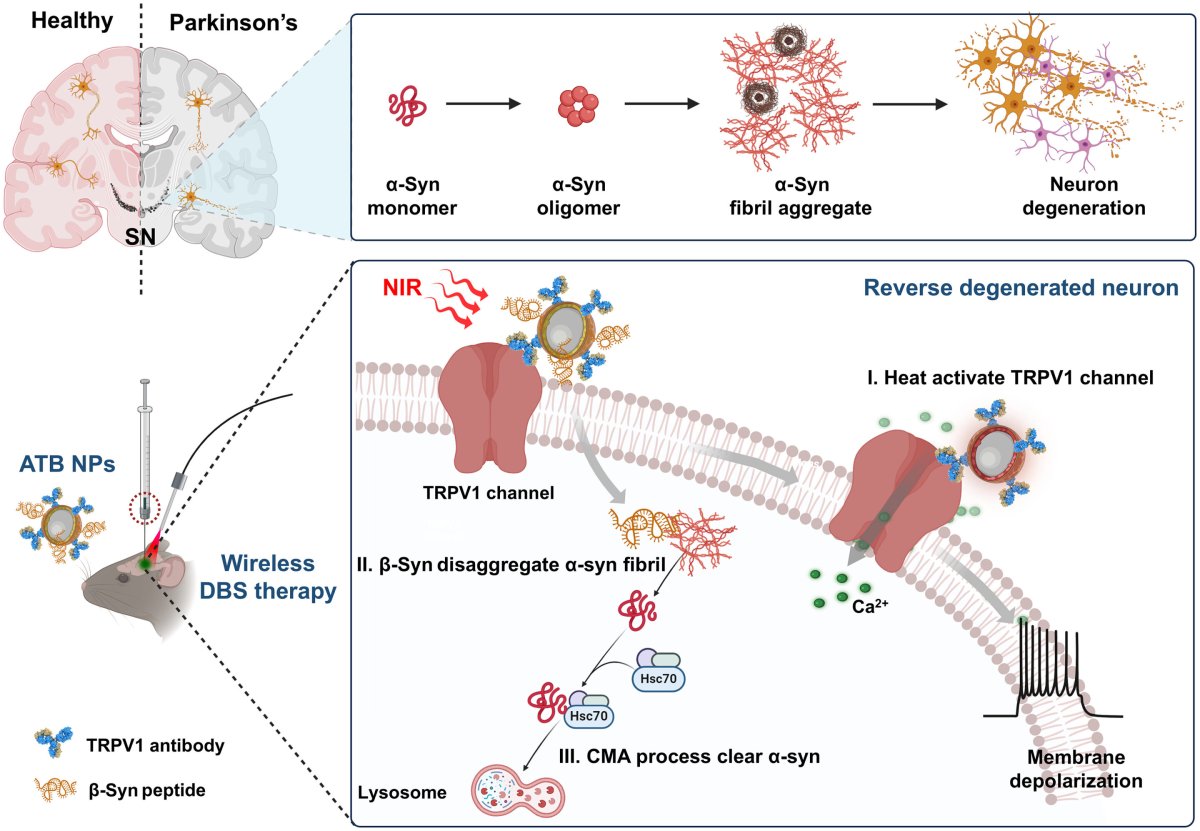
In patients with Parkinson’s disease, a protein called “α-synuclein”—which is normally involved in regulating neurotransmitters—becomes deformed, clumping together into thread-like structures called fibrils and larger masses known as Lewy bodies.
These abnormal build-ups disrupt the function of brain cells, and lead to the degeneration and ultimately death of the neurons that produce the neurotransmitter dopamine, which is involved in movement, memory and motivation.
To combat this, a common treatment—”deep brain stimulation”—sees patients implanted with electrodes in specific regions of the brain. These implants are used to send electrical impulses into the brain to help modulate the activity of the neurons.
The issue with electrode-based deep brain stimulation, however, is that it is inherently invasive; because of this, it can cause both cognitive decline and emotion disturbances like anxiety and depression.
Less invasive alternatives have been developed in recent years, including “transcranial direct current stimulation” (in which a current is applied to the scalp) and “transcranial magnetic stimulation” (where magnetic fields used instead)—but such approaches are limited by how far they can penetrate the brain and a limited spatial resolution.
Science Advances 2025. DOI: 10.1126/sciadv.ado4927
To address these limitations, Professor Chunying Chen of China’s National Center for Nanoscience and Technology and her colleagues have developed an implant-free form of direct brain stimulation based around nanoparticles that are activated by near-infrared laser pulses.
Each nanoshell has three main components: a central gold nanoshell, and coming off this a set of “targeting” and “degradation” arms.
The latter allow the nanoparticles to bind to and destroy harmful α-synuclein fibrils, while the former specifically target a temperature-sensitive receptor in dopamine-producing neurons that are in turn stimulated by the gold nanoshells, which translate the laser light into heat.
In experiments on mice with Parkinson’s disease, the researchers found that the laser-activated nanoparticles restored the rodents’ network of dopamine-producing neurons, improving their motor functions in the process.
The researchers concluded: “This proof-of-concept study provides valuable insights for future investigations aiming to expand the field of direct brain stimulation without the need for additional implantation of conduits or genetic manipulation.”
Do you have a tip on a health story that Newsweek should be covering? Do you have a question about Parkinson’s disease? Let us know via science@newsweek.com.
Reference
Wu, J., Cui, X., Bao, L., Liu, G., Wang, X., & Chen, C. (2025). A nanoparticle-based wireless deep brain stimulation system that reverses Parkinson’s disease. Science Advances, 11(3). https://doi.org/10.1126/sciadv.ado4927
Source link






























